What is driving the “One and Done Syndrome” for PIs (Principal Investigators)? This part 2 of the article brief series explores the challenges of industry requirement standardization, competency domains, PI perceptions and strategies for success.
There are myriad challenges PIs face in conducting clinical trials including:
- Complex trial protocols
- Stringent Sponsor/CRO requirements
- Strict regulations for study conduct
- Involved implementation of new technologies
- Uneven compensation for certain areas of clinical trial conduct such as ongoing trial oversight and review of adverse events
The FDA Requirement 312.53 (a) advises sponsors/CROs to “…select only investigators qualified by training and experience as appropriate experts.” However, there are no guidelines that define the specific training and optimal parameters for experience. This makes it problematic for physicians considering the role of a PI to fully understand the details and intricacies of the position before becoming part of the clinical trial process.
“The FDA investigator requirements are nebulous and difficult to clarify. A solution is to set standards
for required competencies and validate them as an industry,” said Jim Kremidas, Executive Director,
Association of Clinical Research Professionals.
At Harvard University, the MRCT (Multi-Regional Clinical Trials) Center Joint Task Force for Competency Domains has defined 8 areas including:
- Ethical and participant safety considerations
- Medicines development and regulation
- Clinical trial operations and GCP (Good Clinical Practice)
- Study and site management compliance
- Data management and informatics
- Leadership and professionalism
- Communication and teamwork
Delineation of key responsibilities and tasks can furnish clarification, impart points for further discussion and add context to challenges managed by industry professionals.
PIs report a number of significant barriers to continuing with clinical research.
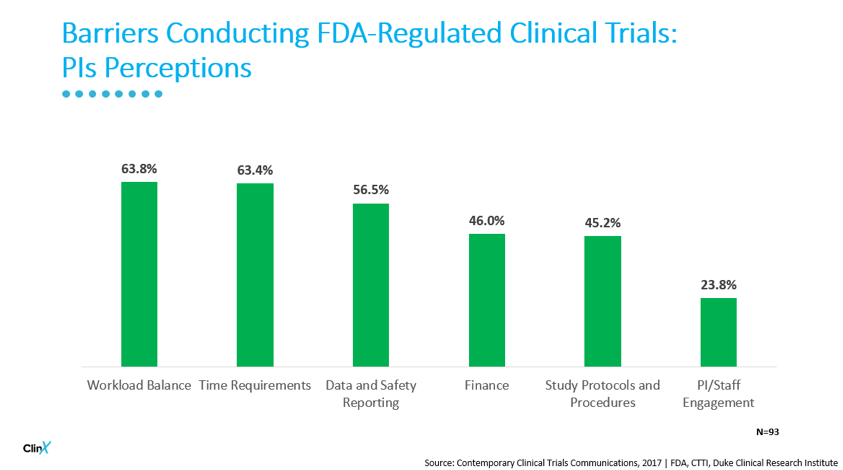
There are other impacts of the “One and Done Syndrome” including:
- Degradation of data quality
- Injudicious management of clinical trial budgets
- Delayed delivery of new therapies to patients in need
“Most people fall into clinical research, they don’t plan to go into it. We can advance the workforce by raising clinical research as a career option to attract new and diverse talent,” offered Jim.
“Another way to drive PI and staff engagement is to provide opportunities for PIs to learn about new studies
and contribute strategic input on protocol design at the outset,” added Joan Chambers, Vice President
of Marketing and Strategy, ClinX.
PIs will have a greater chance of success with an understanding of clinical research demands, the integration of strong work-flow processes and expectations of sponsors/CROs.
Article Brief covers the “Addressing the ‘One and Done’ Syndrome in Clinical Trials” webinar presented by Joan Chambers, VP Marketing & Strategy ClinX.