PIs and the “One and Done Syndrome” In Clinical Trials
Part 1 of a 2-part series
Principal Investigators (PIs) provide vital contributions to the success of clinical trials with responsibilities that span medical and operational efficiencies. Sponsors/CROs logically lean towards skilled PIs with positive track records. There is, however, a shortage of experienced PIs in the pharmaceutical industry due to a high turn-over rate. Data indicates that many physicians engage in a single study and then decline further involvement. What factors are driving this “One and Done Syndrome” in the United States?
“We’re witnessing that in Europe and in other parts of the world, the number of PIs are growing at annual rates substantially higher than in North America,” observed Joan Chambers, Vice President of Marketing and Strategy at ClinX.
PI turn-over is influenced by several key factors including:
- Complex regulatory requirements
- Sponsors placing pressure on PIs to deliver more with less resources
- Narrow patient eligibility requirements resulting in patient recruitment challenges
Sites are experiencing increasing costs due to implementation of new technologies and more stringent HIPPA, ICH and IRB requirements. Meanwhile, study budgets have remained flat while the cost of procedures per subject is increasing. For even the most seasoned PIs, these factors can be overwhelming to manage and adds to additional work strain on site staff.
“The financial pressures can dampen enthusiasm for clinical research for PIs,” said Joan.
“The downside of doing clinical trials is that there are up-front costs that you won’t be compensated for because Sponsors/CROs view them as the site’s responsibility, part of the cost of doing business,” said Elvin Thalund, Director, Industry Strategy, Oracle Health Sciences. “There’s a higher chance of PI success if they are fully informed about the budget and the sponsor is invested in supporting expenses.”
“Another concern is that the experienced PI pool seems to be aging,” explained Joan. “Data from NIH indicates there are not enough new PIs to replace the ones that are retiring.”
“The actual number of PIs globally remains a mystery,” Elvin added. “There are different estimates based on studies made on public databases. For example, data from a Tufts Center for the Study of Drug Development study on CDER’s BMIS database estimates 40,00 active PIs with a 3.3% annual growth rate.”
Clinical trial protocol design and complexity may also be driving the “One and Done Syndrome.” The mean number of distinct procedures per protocol has increased for Phases 1, 2 and 3 but are weighted more towards Phases 2 and 3. In comparison, the number of planned study volunteer visits grew at a more modest rate. That resulted in many procedures being performed per study volunteer, creating a great burden on volunteer participation.
Total cost per study volunteer has grown because of the increase in the total number of procedures actually being performed. Over a 10-year period, Phase 2 studies saw the highest increase in the cost per study volunteer, at about 61%. Phase 1 protocols closely followed with a 49% increase in growth. The rate for Phase 3 was 34%.
In tandem, the collection of data points has escalated dramatically.
“From 2001 to 2005, we were collecting 494,000 data points. Now we’re collecting 929,00 data points, a large increase of 88%,” said Joan. “This reveals the level of responsibility for conducting and managing clinical trials that PIs and site staff face, in addition to other tasks.”
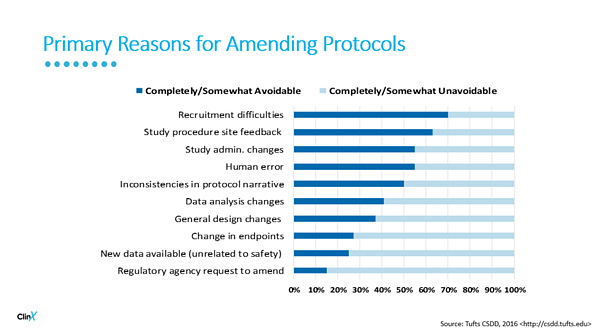
Protocol complexity places challenges on PIs and their colleagues. Protocol amendments must be addressed and for each change, there is an affiliated cost. The chart below outlines common reasons for amending protocols and what could be avoided if proper planning was implemented.
Other barriers that may contribute to PI fatigue include:
- Long, unpredictable work hours
- Finding time to devote to other clinical and non-clinical work activities
- The amount of time required by the PI to prepare for trial start-up
- Study protocol and procedures including patient identification and recruitment
- Study inclusion/exclusion criteria
- Training for their own role and site staff
- Lack of streamlined technology to capture clinical data
- Contract negotiation challenges with Sponsors/CROs and the scheduling of payments
- The amount and frequency of data and safety reporting
The industry is evolving and there is an increasing focus on environmental development and the “One and Done Syndrome”. Consideration must be given to how research sites will change and operate in the future. Workforce competencies will need to expand as technology starts to play a more integral role in the conduct of clinical research and clinical trials become a routine care option.
“Looking to the future, living rooms could become the next clinical trial site for specific studies,” commented Joan.
Patient services are becoming increasingly virtual and automated in nature with remote medical device monitoring, video consultations, and even consultations via chatbot. The improvement in mobile devices used in clinical trials is addressing challenges with security, cost, data validation, FDA acceptance and patient compliance.